You will need to use the Activity Series and the Solubility Chart found in your book. Two examples are also sho.

Writing And Balancing Reactions Double Replacement Youtube
Nitric acid lithium sulfide.

. A double replacement reaction is represented by the general equation. Select two compounds above and this calculator will predict whether or not the reaction will occur in water. When properly balanced the sum of the balancing coefficients is A 7 B 6 C 9 D 8 1 2 The following reaction.
Describes the basics of double replacement reactions how to identify them predict the products and balance the chemical equation. Most chemical reactions fall into one of five general reaction types. There are three different ways to write a balanced chemical equation to describe a double displacement reaction.
Balance the following equations and indicate the type of reaction taking place. The molecular equation the complete ionic equation and the net ionic equation. Predict if these reactions will occur.
DOUBLE REPLACEMENT PRACTICE REACTIONS For each reaction predict the products and balance the equation. Single Displacement Reactions. Acid-base g Whats the main difference between a double displacement reaction and an acid-base reaction.
How do you balance a double displacement reaction. Na 1 aq Br 1- aq H 1 aq Cl 1- aq. AlOH3 Type of reaction.
HBr Type of reaction. Many double displacement reactions occur between ionic compounds that are dissolved in water. Pin On Miss Chemistry Double Displacement Reactions Worksheets - Kiddy Math A double displacement reaction is an important type of chemical reaction to know when studying chemistry.
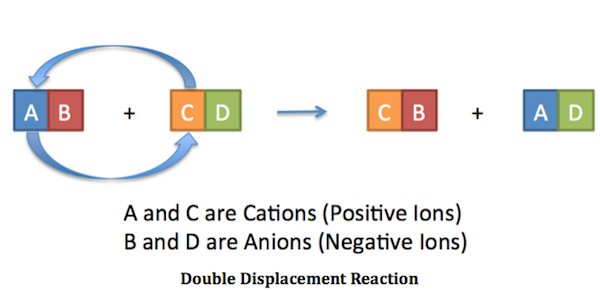
Switch the Cations and Anions of the reactants. If yes complete and balance the equation. Predict the products and write balanced equations for the following double displacement reactions.
This is simply based on the solubility chart of inorganic compounds. For the reactant Na 2 S there is a cation. Examples of double displacement reaction.
If you do not predict a reaction will occur write No Reaction 1. To illustrate heres an example of a double displacement reaction. Ionic charges are not yet supported and will be ignored.
Balance the Chemical Reaction. Br2 2KI I2 2KBr is an example of A Decomposition B Combination C Single-displacement D Double-displacement 2 3 The following reaction. State the reaction in chemical formulas and in symbols.
H Complete combustion reactions always result in the formation of water. A Na B OH. Nickel II chloride potassium bromide.
Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds. In themolecular equationall the reactants and products are represented as neutral chemical compounds. Acid-base reactions form water.
Identify the Individual Ions from the Reactants and Their Charges. If you are interested in a simplified way of balancing double replacement reaction you can balance A B C and D meaning handling the cations and anions as a group rather than as individual elements. Fe Au Co Br C O N F.
Writing and Balancing Equations II Balance each double displacement reaction including the phases only if a reaction will occur. The overall pattern of a double replacement reaction looks like this. Lithium carbonate magnesium bromide.
Double displacement 3 3 Mg 1 Fe 2O3 2 Fe 3 MgO Type of reaction. Use uppercase for the first character in the element and lowercase for the second character. AgNO 3 NaCl --- Silver nitrate sodium chloride silver chloride sodium nitrate AgNO 3 NaCl --- AgCl NaNO 3 1 CaOH 2 H 3 PO 4--- 2 K 2 CO 3 BaCl 2--- 3 Cd 3 PO4 2 NH 4 2.
Barium hydroxide hydroiodic acid. Double displacement 2 3 CaOH 2 1 Al 2SO 43 3 CaSO 4 2 AlOH 3 Type of reaction. A double displacement reaction is a type of reaction in which two reactants exchange ions to form two new compounds.
NaOH HClO4 NaClO4 H2O In this question you must recognize that perchlorate ClO4- and hydroxide OH- are polyatomic ions and will not break apart. The balanced equation will appear above. Balance the following equations and indicate the type of reaction taking place.
Up to 24 cash back Types of Reactions Worksheet Solutions. Double Replacement Reactions - Displaying top 8 worksheets found for this concept. Single displacement 4 1 C2H4 3 O2 2 CO 2 2 H2O Type of reaction.
Here are some examples of. Types of reactions - double and single displacement. A double displacement reaction also known as a double replacement reaction or metathesis is a type of chemical reaction where two compounds react and the positive ions cation and the negative.
MgO Type of reaction. It must be noted that the results are not 100. NaOH FeCl_2 - FeOH_2 NaCl The groups of ions are.
The prediction and balancing of these reactions isnt too much of a challenge for students at this stage however they now must consider whether or not each product will remain as part of the aqueous solution or will form a precipitate. Iron II sulfate sodium phosphate. CaOH2 HCl CaCl2 2H2O is an example of.
Ammonium permanganate lithium hydroxide. Transcribed image text. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button.
Double displacement f 3 HBr 1 AlOH 3 3 H-2O 1 AlBr 3 Type of reaction. 6 H 2 SO 4 aq NaOHaq 7 2KIaq PbNO32aq. 1 3 NaBr 1 H3PO 4 1 Na 3PO 4 3 HBr Type of reaction.
Also this is an acid-base reaction so the products should be salt and water. Balancing Equations and Identifying whether reaction is single or double displacement. No DR Reaction A double replacement reaction will occur if a formation of a precipitate gas or water takes place.
Double-displacement reactions involve the switching of cations between two compounds. 1 Als Fe 2 O 3s 2 Mgs H2Og 3 Mgs PbSs 4 Bis H2Og 5 Cos HClaq Double Displacement Reactions.

Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com

Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com

Double Replacement Reaction Practice Problems Examples Youtube

Double Replacement Double Displacement Reaction
How Do You Balance Double Replacement Reactions Socratic

Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com
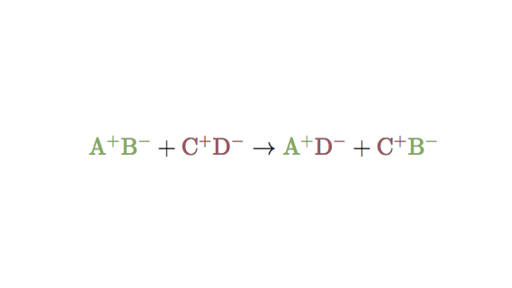
Double Replacement Reactions Double Displacement Article Khan Academy

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube
0 comments
Post a Comment